Patrón de flujo en biorreactores: Rushton, Pitched-Blade e Hydrofoil
En un biorreactor agitado, el patrón de flujo está determinado por el diseño del impulsor. Algunas de las configuraciones más comunes son la turbina Rushton, el impulsor de palas inclinadas (pitched-blade) y el impulsor tipo hydrofoil.
Cada uno genera un movimiento distinto: el impulsor Rushton impulsa el líquido lateralmente (flujo radial), las palas inclinadas combinan movimiento axial y radial, y el hydrofoil impulsa en dirección axial. Estas diferencias en la dirección del flujo influyen directamente en la mezcla, la dispersión de burbujas y la cizalla local.
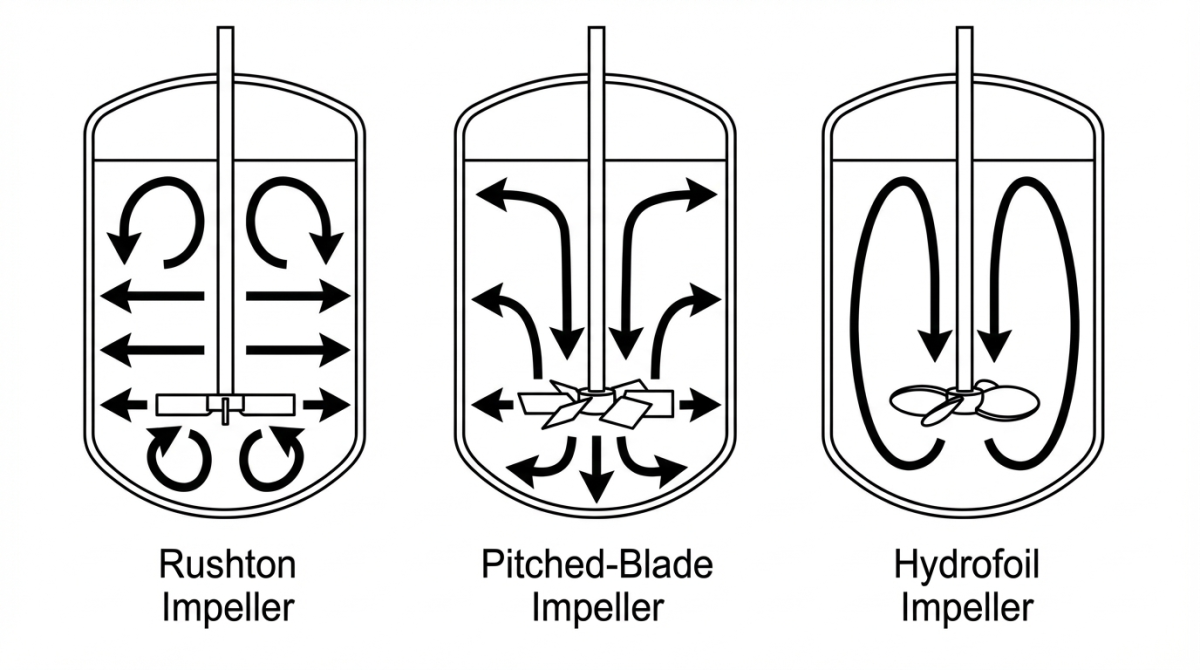
Impulsor Rushton: Flujo radial y alta cizalla
La turbina Rushton es un disco plano con 4–6 palas verticales. Su patrón de flujo es principalmente radial, lo que significa que el líquido se empuja hacia las paredes del tanque. Este chorro lateral genera aproximadamente la mitad del flujo hacia arriba y la otra mitad hacia abajo.
Como resultado, la turbina Rushton produce una intensa cizalla local, es decir, altas fuerzas cortantes cerca de cada pala. Además, sus palas planas son muy efectivas para romper burbujas de gas, lo que incrementa considerablemente la superficie de contacto entre el gas y el líquido. Todo esto se traduce en valores de kLa muy altos, lo que significa una excelente transferencia de oxígeno, aunque a costa de un mayor consumo de energía.
Los rasgos característicos del impulsor Rushton se resumen en:
- Patrón de flujo radial: El flujo se distribuye hacia los costados del reactor.
- Cizalla alta: Las palas planas generan fuertes turbulencias locales.
- Transferencia de oxígeno (kLa) muy alta: Rompe eficazmente las burbujas de gas, alcanzando los kLa mayores.
- Uso típico: Óptima en fermentaciones microbianas (p.ej. E. coli, levaduras) tolerantes a la cizalla. Se prefiere donde la prioridad es la oxigenación sobre la delicadeza celular.
Impulsor pitched-blade: Flujo mixto axial-radial
El impulsor de palas inclinadas (pitched-blade) consta de 4–6 palas planas inclinadas unos 45° respecto al eje. Este diseño produce un flujo mixto axial-radial. Parte del fluido se mueve hacia arriba o hacia abajo (axial) y otra parte se desplaza hacia afuera (radial). El componente axial ayuda a elevar el fluido, mientras que el radial provoca cierta recirculación lateral. En la práctica, esto resulta en un flujo combinado que mezcla bien el medio, logrando un equilibrio entre el bombeo vertical y la turbulencia.
Los aspectos clave del pitched-blade son:
- Patrón de flujo mixto: Combina flujo vertical y radial, reduciendo zonas muertas en el reactor.
- Cizalla moderada: Menor que el impulsor Rushton, ya que el flujo axial amortigua el esfuerzo mecánico sobre las células.
- Transferencia de oxígeno buena: Mantiene una dispersión de gas eficaz, aunque típicamente con kLa algo menor que Rushton a igual potencia.
- Uso típico: Ideal para cultivos celulares sensibles (CHO, HEK, células de mamífero o insectos) que requieren buena mezcla sin un exceso de cizalla. También se emplea en procesos microbianos moderados y en mezclas líquido-líquido.

Impulsor hydrofoil: Flujo axial y mezcla de baja cizalla
El impulsor hydrofoil (o hélice hidrodinámica) tiene generalmente 3 a 4 palas curvas de perfil aerodinámico. El diseño de este sistema está pensado para crear un flujo principalmente axial, especialmente hacia abajo (down-pumping). Esto permite una recirculación vertical del líquido muy eficiente, utilizando muy poca energía. Gracias a la forma curva de las palas, el hydrofoil produce una cizalla mínima en comparación con otras geometrías. A bajas RPM, es capaz de mover grandes volúmenes de fluido, lo que ayuda a mantener la viabilidad de células delicadas.
Sus características clave son:
- Flujo axial fuerte: Bombea el líquido verticalmente con eficiencia (flujo arriba/abajo).
- Muy baja cizalla: Reduce las fuerzas cortantes, lo que es ideal para cultivos sensibles.
- Alta eficiencia energética: Tiene un bajo número de potencia (Po), gran capacidad de bombeo y consumo mínimo de energia.
- Transferencia de oxígeno: Aunque su kLa absoluto puede ser menor que el del impulsor Rushton, el alto caudal de recirculación mantiene una oxigenación efectiva.
- Uso típico: Recomendado en cultivos muy delicados o medios viscosos (hasta decenas de miles de cP), en reactores de un solo uso y para escalado suave donde se minimiza el esfuerzo mecánico.

Comparación de patrones de flujo y rendimiento en biorreactores
Las diferencias se pueden resumir en la siguiente comparación. La dirección del flujo es distinta: la Rushton genera un flujo principalmente radial (hacia los lados), el pitched-blade produce un flujo mixto axial-radial, y el hydrofoil impulsa el líquido de manera axial (vertical). Además, la cizalla inducida es más alta con la Rushton, intermedia con el pitched-blade y baja con el hydrofoil.
En cuanto al rendimiento en la transferencia de oxígeno, también hay variaciones: la Rushton rompe las burbujas de manera muy eficiente (con un kLa muy alto), mientras que los impulsores axiales logran un kLa un poco más bajo a la misma potencia, pero mantienen una buena oxigenación gracias a una alta recirculación. Por último, el consumo energético es mayor en la Rushton (Po≈5–6), medio en el pitched-blade (Po≈2–3) y menor en el hydrofoil (Po≈1–1.2).
En resumen, los impulsores axiales (pitched-blade/hydrofoil) logran una mezcla más suave y eficiente por volumen impulsado, mientras que la Rushton proporciona la máxima dispersión de gas.
Cizalla, mezcla y transferencia de oxígeno en los patrones de flujo de biorreactores
En términos generales, los impulsores de flujo axial, como los de palas inclinadas y los hydrofoil, logran una mezcla más intensa que los radiales. Esto se traduce en mezclas homogéneas más rápidas con la misma potencia, además de reducir las áreas de cizalla extrema. Por otro lado, el impulsor Rushton genera turbulencias amplias que favorecen la dispersión de gas. Por ejemplo, todos los estudios coinciden en que los impulsores axiales superan a los radiales en cuanto a la intensidad de mezcla.
En lo que respecta al oxígeno disuelto, el Rushton ofrece los kLa más altos gracias a la ruptura violenta de burbujas, pero los impulsores axiales pueden compensar esto mediante una mejor circulación del fluido. En la práctica, con potencias similares, el Rushton suele tener la ventaja en kLa, mientras que el hydrofoil logra una buena oxigenación con un menor consumo energético al mantener un gran recirculado.

Suspensión celular y aplicaciones recomendadas
La elección del impulsor también depende del tipo de cultivo que estés manejando. Los organismos microbianos más robustos, como E. coli y las levaduras, son bastante resistentes a la alta cizalla, por lo que se suelen utilizar turbinas Rushton para maximizar la transferencia de oxígeno. Por otro lado, los cultivos más sensibles, como las células de mamífero o de insecto, requieren mezclas más suaves; en estos casos, los impulsores axiales son la mejor opción. Los impulsores de palas inclinadas y hydrofoil son excelentes para asegurar una buena circulación mientras mantienen baja la cizalla local.
Por ejemplo, las células CHO o HEK tienden a proliferar mejor con palas inclinadas o hydrofoil, ya que estos diseños ayudan a preservar la viabilidad celular al generar menos turbulencia. Para cultivos que son viscosos o de gran escala, el hydrofoil resulta ser especialmente útil: su diseño permite el manejo de fluidos de alta viscosidad (hasta aproximadamente 50,000 cP) y proporciona altos caudales con un esfuerzo mínimo. En contraste, los procesos mixtos o secuenciales pueden beneficiarse de la combinación de impulsores, como tener un impulsor de palas inclinadas arriba y un Rushton abajo, para equilibrar la mezcla y la oxigenación según sea necesario.
Tabla: comparación de los patrones de flujo de los impulsores más comunes en biorreactores
| Característica | Rushton (turbina radial) | Pitched-blade (PBT) | Hydrofoil |
|---|---|---|---|
| Patrón de flujo (dominante) | Radial (chorro horizontal intenso) | Mixto axial–radial (descarga diagonal) | Axial (bombeo vertical dominante) |
| Circulación típica en STR con bafles | Dos bucles toroidales (uno arriba y otro abajo del impulsor) | Un bucle grande, depende de down- vs up-pumping | Bucle vertical “limpio”, depende de down- vs up-pumping |
| Lo que mejor hace | Rotura de burbuja y dispersión de gas | Mezcla “todoterreno” del volumen | Gran recirculación con poca potencia |
| Cizalla cerca de las palas | Alta | Moderada | Baja |
| Dispersión de gas / kLa (típico) | Muy alta | Buena (suele ser menor que Rushton a igual potencia) | Buena respecto a la potencia (eficiente) |
| Eficiencia energética (tendencia Po) | Baja (Po ~5–6) | Media (Po ~2–3) | Alta (Po ~1–1.2) |
| Casos de uso típicos | Fermentaciones microbianas aeróbicas (alta demanda de O₂) | Cultivo celular y mezcla general | Cultivos sensibles, escalado suave, medios viscosos |
Cómo elegir el patrón de flujo adecuado para tu biorreactor
En un biorreactor STR, el patrón de flujo es una variable de proceso que condiciona la mezcla real del tanque, la dispersión de gas, el kLa alcanzable y, sobre todo, la exposición del cultivo a cizalla.
Si quieres profundizar en la selección del impulsor más allá del movimiento del fluido, te recomendamos el otro artículo de la serie, centrado en comparar Rushton, pitched-blade e hydrofoil desde el punto de vista de kLa, consumo energético, cizalla y criterios de escalado, para ayudarte a decidir con datos y no solo por “reglas rápidas”.
En este contexto, TECNIC ofrece biorreactores de un solo uso y de acero inoxidable multi-use configurables con turbinas Rushton y pitched-blade, lo que permite adaptar el sistema de agitación al tipo de proceso (microbiano o celular) y a la estrategia de escalado. Si necesitas validar qué patrón de flujo y configuración encajan mejor con tu cultivo, nuestro equipo puede asesorarte para definir la geometría y el sistema de agitación más adecuado para tu aplicación.

Frequently asked questions about flow patterns in stirred-tank bioreactors
In an STR, the flow pattern is the dominant circulation path created by the impeller inside the vessel. It describes how liquid moves (axial, radial or mixed), which directly affects mixing time, gas dispersion, local shear and how quickly the whole tank becomes homogeneous.
Radial flow pushes liquid sideways toward the tank wall (strong horizontal jet). Axial flow pumps liquid mainly up or down along the vessel axis (strong vertical circulation). Mixed flow combines both components, typically with a diagonal discharge that improves bulk circulation while maintaining some radial mixing.
A Rushton turbine is predominantly radial-flow. It generates a strong horizontal jet that hits the vessel wall and splits into two circulation loops (one above and one below the impeller), especially in baffled tanks. This pattern is typically associated with strong gas dispersion and high local turbulence.
A pitched-blade turbine produces mixed axial–radial flow. The discharge leaves the blades diagonally, so the impeller can pump up or down (depending on blade orientation), while still generating a radial component that helps distribute flow across the vessel diameter.
Hydrofoil impellers are mainly axial-flow designs. They are optimised to move large liquid volumes vertically (strong pumping) with relatively low power input, typically creating a clean vertical circulation loop that supports efficient bulk mixing at lower local shear.
Baffles (typically 3–4 vertical plates) reduce swirl and suppress vortex formation, so more of the impeller power is converted into a defined flow pattern (axial, radial or mixed) instead of “spinning” the whole liquid volume. In baffled STRs, circulation loops become more stable, mixing time usually improves, and gas dispersion tends to be more consistent. Without baffles, strong tangential motion can dominate, leading to poor top-to-bottom exchange, surface vortexing and less predictable oxygen transfer.
Yes. Blade orientation determines whether the impeller pumps liquid downward or upward, which changes where high-velocity zones form and how quickly the top and bottom of the tank exchange fluid. Down-pumping is often preferred for surface-to-bottom circulation and gas handling, while up-pumping can be useful in specific suspension or surface renewal scenarios.
Axial and mixed-flow impellers typically improve top-to-bottom circulation and reduce stagnant regions, especially in taller tanks. Radial turbines can mix efficiently near the impeller zone but may require multiple impellers or specific placement to avoid stratification in large volumes.
Gas dispersion depends on how the impeller interacts with bubbles and where gas is carried in the vessel. Radial turbines often break bubbles efficiently and can deliver high kLa at higher power. Axial designs can maintain effective oxygenation by sustaining strong circulation and distributing bubbles throughout the working volume, depending on sparger and gas rate.
Multiple impellers are common in tall vessels, higher viscosity media or large-scale STRs where one impeller cannot circulate the entire height effectively. Adding a second (or third) impeller helps stabilise axial circulation, reduce stratification and improve overall gas and nutrient distribution across the full liquid column.
Referencias
- Fluid Flow and Mixing With Bioreactor Scale-Up – Bioprocess International. Explains how impellers generate axial and radial circulation patterns and how these affect mixing and mass transfer during scale-up.
- A Review of Stirred Tank Dynamics: Power Consumption, Mixing Time and Impeller Geometry – ResearchGate. Technical review covering stirred-tank hydrodynamics, flow patterns and the impact of impeller geometry on mixing performance.
- Stirred-Tank Bioreactors – ScienceDirect Topics. Engineering overview of flow behaviour and mixing patterns in stirred-tank bioreactors.
Este artículo presenta un análisis técnico y basado en datos de los impulsores para biorreactores, comparando los diseños Rushton, pitched-blade e hydrofoil desde la perspectiva de los patrones de flujo, la transferencia de oxígeno (kLa), el entorno de cizalla y la eficiencia energética en biorreactores agitados de escala laboratorio, piloto y producción. El contenido está estructurado para ayudar a entender cómo el flujo generado por el impulsor influye en el comportamiento de la mezcla y cómo estas diferencias afectan al rendimiento del proceso y a las decisiones de escalado.
Este artículo ha sido revisado y publicado por TECNIC Bioprocess Solutions, fabricante de biorreactores agitados escalables, sistemas de filtración de flujo tangencial y consumibles de un solo uso para el desarrollo de bioprocesos, operaciones piloto y fabricación bajo GMP.







